Environmental Cracking: HB, HIC, SWC, SOHIC, SSC, SZC, HSC, HE, SCC
Introduction
In industries where materials are subjected to harsh environments—such as oil and gas, chemical processing, and power generation—understanding and preventing environmental cracking is critical. These types of cracking can lead to catastrophic failures, costly repairs, and significant safety risks. This blog post will provide a detailed and professional overview of the various forms of environmental cracking like HB, HIC, SWC, SOHIC, SSC, SZC, HSC, HE, and SCC, including their recognition, underlying mechanisms, and strategies for prevention.
1. Hydrogen Blistering (HB)
Recognition:
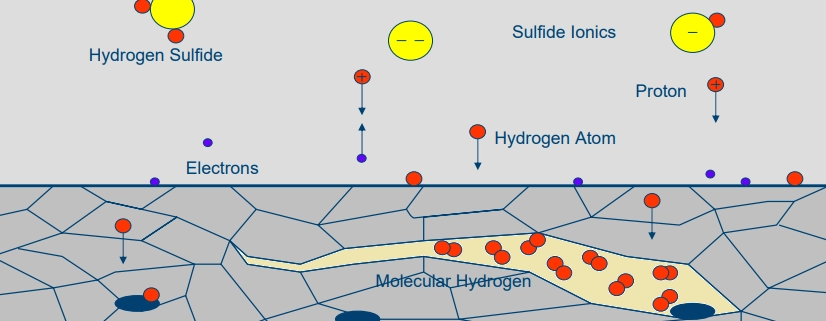
Hydrogen blistering is characterized by the formation of blisters or bulges on the surface of a material. These blisters result from hydrogen atoms penetrating the material and accumulating at internal defects or inclusions, forming hydrogen molecules that create localized high pressure.
Mechanism:
Hydrogen atoms diffuse into the material, typically carbon steel, and recombine into molecular hydrogen at sites of impurities or voids. The pressure from these hydrogen molecules creates blisters, weakening the material and leading to further degradation.
Prevention:
- Material Selection: Use low-impurity materials, particularly steels with low sulfur content.
- Protective Coatings: Application of coatings that prevent hydrogen ingress.
- Cathodic Protection: Implementation of cathodic protection systems to reduce hydrogen absorption.
2. Hydrogen-Induced Cracking (HIC)
Recognition:
Hydrogen-induced cracking (HIC) is identified by internal cracks that often run parallel to the material’s rolling direction. These cracks are typically located along grain boundaries and do not extend to the material’s surface, making them difficult to detect until significant damage has occurred.
Mechanism:
Like hydrogen blistering, hydrogen atoms enter the material and recombine to form molecular hydrogen within internal cavities or inclusions. The pressure generated by these molecules causes internal cracking, compromising the material’s structural integrity.
Prevention:
- Material Selection: Opt for low-sulfur steels with reduced levels of impurities.
- Heat Treatment: Employ proper heat treatment processes to refine the material’s microstructure.
- Protective Measures: Use coatings and cathodic protection to inhibit hydrogen absorption.
3. Stress-Oriented Hydrogen-Induced Cracking (SOHIC)
Recognition:
SOHIC is a form of hydrogen-induced cracking that occurs in the presence of external tensile stress. It is recognized by a characteristic stepwise or staircase-like crack pattern, often observed near welds or other high-stress areas.
Mechanism:
Hydrogen-induced cracking and tensile stress lead to a more severe and distinct cracking pattern. The presence of stress exacerbates the effects of hydrogen embrittlement, causing the crack to propagate stepwise.
Prevention:
- Stress Management: Implement stress-relief treatments to reduce residual stresses.
- Material Selection: Use materials with higher resistance to hydrogen embrittlement.
- Protective Measures: Apply protective coatings and cathodic protection.
4. Sulfide Stress Cracking (SSC)
Recognition:
Sulfide stress cracking (SSC) manifests as brittle cracks in high-strength steels exposed to hydrogen sulfide environments (H₂S). These cracks are often intergranular and can propagate rapidly under tensile stress, leading to sudden and catastrophic failure.
Mechanism:
In the presence of hydrogen sulfide, hydrogen atoms are absorbed by the material, leading to embrittlement. This embrittlement reduces the material’s ability to withstand tensile stress, resulting in brittle fracture.
Prevention:
- Material Selection: Use of sour-service-resistant materials with controlled hardness levels.
- Environmental Control: Reducing exposure to hydrogen sulfide or using inhibitors to minimize its impact.
- Protective Coatings: Application of coatings to act as barriers against hydrogen sulfide.
5. Stepwise Cracking (SWC)
Recognition:
Stepwise or hydrogen cracking occurs in high-strength steels, particularly in welded structures. It is recognized by a zigzag or staircase-like crack pattern, typically observed near welds.
Mechanism:
Stepwise cracking occurs due to the combined effects of hydrogen embrittlement and residual stress from welding. The crack propagates stepwise, following the weakest path through the material.
Prevention:
- Heat Treatment: Use pre- and post-weld heat treatments to reduce residual stresses.
- Material Selection: Opt for materials with better resistance to hydrogen embrittlement.
- Hydrogen Bake-Out: Implement hydrogen bake-out procedures after welding to remove absorbed hydrogen.
6. Stress Zinc Cracking (SZC)
Recognition:
Stress zinc cracking (SZC) occurs in zinc-coated (galvanized) steels. It is recognized by intergranular cracks that can lead to the delamination of the zinc coating and subsequent structural failure of the underlying steel.
Mechanism:
The combination of tensile stress within the zinc coating and exposure to a corrosive environment causes SZC. The stress within the coating, coupled with environmental factors, leads to intergranular cracking and failure.
Prevention:
- Coating Control: Ensure proper zinc coating thickness to avoid excessive stress.
- Design Considerations: Avoid sharp bends and corners that concentrate stress.
- Environmental Control: Reduce exposure to corrosive environments that could exacerbate cracking.
7. Hydrogen Stress Cracking (HSC)
Recognition:
Hydrogen stress cracking (HSC) is a form of hydrogen embrittlement in high-strength steels exposed to hydrogen. It is characterized by sudden brittle fracture under tensile stress.
Mechanism:
Hydrogen atoms diffuse into the steel, causing embrittlement. This embrittlement significantly reduces the material’s toughness, making it prone to cracking and sudden failure under stress.
Prevention:
- Material Selection: Choose materials with lower susceptibility to hydrogen embrittlement.
- Environmental Control: Minimize hydrogen exposure during processing and service.
- Protective Measures: Use protective coatings and cathodic protection to prevent hydrogen ingress.
8. Hydrogen Embrittlement (HE)
Recognition:
Hydrogen embrittlement (HE) is a general term for the loss of elasticity and subsequent cracking or fracture of a material due to hydrogen absorption. The sudden and brittle nature of the fracture is often recognized.
Mechanism:
Hydrogen atoms enter the metal’s lattice structure, significantly reducing its ductility and toughness. Under stress, the embrittled material is prone to cracking and failure.
Prevention:
- Material Selection: Use materials that are resistant to hydrogen embrittlement.
- Hydrogen Control: Manage hydrogen exposure during manufacturing and service to prevent absorption.
- Protective Coatings: Apply coatings that prevent hydrogen from entering the material.
9. Stress Corrosion Cracking (SCC)
Recognition:
Stress corrosion cracking (SCC) is characterized by fine cracks that typically initiate at the material’s surface and propagate through its thickness. SCC occurs when a material is exposed to a corrosive environment under tensile stress.
Mechanism:
SCC results from the combined effects of tensile stress and a corrosive environment. For instance, chloride-induced SCC is a common issue in stainless steels, where chloride ions facilitate crack initiation and propagation under stress.
Prevention:
- Material Selection: Choose materials resistant to specific types of SCC relevant to the environment.
- Environmental Control: Reduce the concentration of corrosive species, such as chlorides, in the operating environment.
- Stress Management: Use stress-relief annealing and careful design to minimize residual stresses contributing to SCC.
Conclusion
Environmental cracking represents a complex and multifaceted challenge for industries where material integrity is critical. Understanding the specific mechanisms behind each type of cracking—such as HB, HIC, SWC, SOHIC, SSC, SZC, HSC, HE, and SCC—is essential for effective prevention. By implementing strategies like material selection, stress management, environmental control, and protective coatings, industries can significantly reduce the risks associated with these forms of cracking, ensuring the safety, reliability, and longevity of their infrastructure.
As technological advancements continue to evolve, so too will the methods for combating environmental cracking. This makes ongoing research and development vital to maintaining material integrity in ever-demanding environments.