Does Steel Rust? An In-depth Analysis
Steel is one of the most widely used materials in industries ranging from oil and gas to chemical processing and marine engineering. Known for its strength, versatility, and cost-effectiveness, steel is an essential component in countless applications. However, one common question that arises when working with steel is: Does Steel Rust? —and, if so, why.
While steel is an alloy of iron and carbon, its behavior in different environments can vary. Understanding how and why steel rusts, and more importantly, how to prevent it, is crucial for professionals working in demanding industries where equipment and infrastructure must withstand harsh conditions. This blog post will explore why steel rusts, the factors influencing rusting, and the best ways to mitigate corrosion in the oil and gas, chemical processing, and marine engineering sectors.
1. What is Steel? Why Does Steel Rust?
Steel is an alloy primarily made of iron with a small amount of carbon. It’s used in various applications due to its exceptional mechanical properties such as strength, toughness, and ductility. However, iron itself is highly susceptible to corrosion when exposed to moisture and oxygen.
Rusting occurs when iron reacts with oxygen and water in the environment, forming iron oxide. This reaction is a type of oxidation; over time, it weakens the steel and can cause it to deteriorate.
While steel’s carbon content makes it stronger and more durable than pure iron, it doesn’t significantly change its susceptibility to rust. The more carbon in the steel, the more prone it may be to cracking under stress, but the rusting process remains unchanged mainly unless the steel is treated or alloyed with other materials.
2. Factors That Cause Steel to Rust
Rust is not a universal outcome for all steel; certain factors contribute to its occurrence. Here are the primary causes of steel rust:
a. Exposure to Moisture
Moisture is one of the main contributors to rust. Water accelerates oxidation, especially when it contains dissolved salts or minerals. Steel that is frequently exposed to humid air, rain, or water may begin to rust faster than steel kept dry.
b. Oxygen
Another key factor is the presence of oxygen. When iron or steel is exposed to oxygen, it forms iron oxide (rust). However, without oxygen, the rusting process cannot proceed. This is why steel can be stored or maintained without rusting in environments where oxygen is excluded (e.g., under certain coatings or submerged in oil).
c. Salts and Chemicals
Salt, particularly saltwater, is a significant catalyst for rusting. In coastal areas, steel structures exposed to seawater can corrode rapidly due to the high chloride content in the water. Similarly, certain chemicals in industrial settings, such as acids and alkalis, can also contribute to the breakdown of the protective surface of steel.
d. Temperature
Heat can accelerate the rusting process. High temperatures increase the rate of chemical reactions, including oxidation. This is particularly true in environments like chemical processing or oil refineries where equipment is exposed to intense heat for extended periods.
e. Airborne Pollutants
In industrial settings, airborne pollutants such as sulfur dioxide, carbon dioxide, and other gases can react with water or moisture in the air to form acids, which in turn can corrode steel. These pollutants are particularly common in urban environments or industrial plants.
3. What is the Mechanism of Steel Rusting?
Corrosion is the process by which a material, usually metal, gradually degrades or destroys through electrochemical reactions with its surroundings. In the case of metals, corrosion is the process of rusting – an oxidation reaction with oxygen to form iron oxide. Corrosion requires both water and oxygen; if either is absent, corrosion will not occur.
The steel corrosion process occurs in stages. Initially, corrosion begins at the anodic region of the surface, where ferrous ions enter the solution. Electrons are released from the anodic surface and travel through the metal structure to the adjacent cathodic site, where they combine with oxygen and water to form hydroxyl ions. These hydroxyl ions react with the ferrous ions at the anodic site to form ferrous hydroxide, which itself further oxidizes in air to form hydrated ferric oxide (rust). The following equation can represent the chemical process:
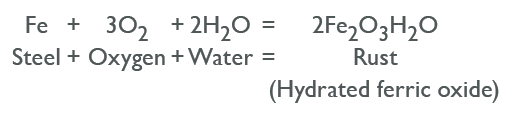
Equation of How Does Steel Rust
Over time, the buildup of rust on the surface inhibits the corrosion process. New anodic sites may form in adjacent areas, leading to further corrosion. In this case, the metal loss is fairly uniform across the surface over a long period, often described as general corrosion or uniform corrosion. Anodic sites release electrons, and cathodic sites receive them, forming the basis of an electrical circuit that drives the corrosion process.
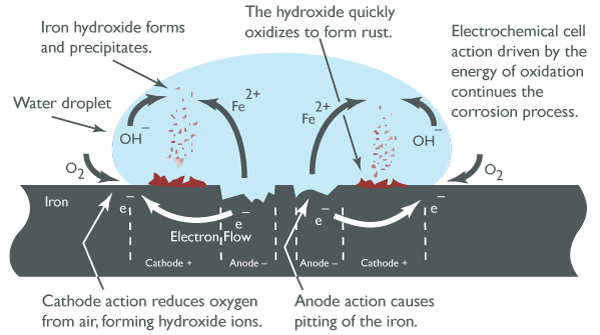
Mechanism of Steel Rusting
4. Types of Steel and Rust Resistance
Different types of steel exhibit varying degrees of resistance to rust. The key factor influencing rust resistance in steel is the presence of alloying elements such as chromium, nickel, and molybdenum, which help to form a protective oxide layer on the surface of the steel. Here are some common types of steel and their rust resistance:
a. Carbon Steel
Carbon steel, the most basic and common form of steel, is highly susceptible to rust when exposed to moisture and oxygen. It is cheap and versatile but requires protective coatings or regular maintenance to prevent rusting in harsh environments.
b. Stainless Steel
Stainless steel contains at least 10.5% chromium, which forms a thin, protective layer of chromium oxide on the surface, preventing further oxidation. While stainless steel is resistant to rust, it can still corrode under extreme conditions (such as exposure to saltwater or certain acids). Stainless steel grades such as 316 or 304 are often used in marine environments due to their superior corrosion resistance.
c. Galvanized Steel
Galvanized steel is carbon steel that has been coated with a layer of zinc. The zinc coating protects the steel from rusting by acting as a sacrificial anode. Even if the coating is damaged, the zinc will corrode before the steel does, offering a degree of protection. However, galvanized steel is still susceptible to rust over time, especially in environments with high humidity or chemical exposure.
d. Alloy Steel
Alloy steel contains additional elements like chromium, nickel, or molybdenum that improve its corrosion resistance. Depending on the alloy content, these steels can be highly resistant to rust in various environments, making them suitable for harsh industrial applications such as chemical processing or oil and gas extraction.
5. How Does Steel Rust Affect Industries that Use Steel Products?
Rusting is particularly problematic in industries that rely on steel for infrastructure and equipment exposed to extreme conditions. Let’s break down the implications of rust in oil and gas, chemical processing, and marine engineering.
a. Oil & Gas Industry
Steel is widely used in pipelines, pressure vessels, and drilling rigs in the oil and gas sector. However, these structures are exposed to corrosive environments, including hydrogen sulfide (H2S) and saltwater. Rust can cause leaks, reduce the integrity of pipes, and lead to costly maintenance or catastrophic failures. Materials with higher corrosion resistance, such as alloy steel or coated carbon steel, are often used to combat this. Regular inspections and maintenance are also essential to prevent rust from compromising safety.
b. Chemical Processing Industry
Steel components in chemical processing plants are exposed to aggressive chemicals, extreme temperatures, and high pressures. Rusting in these conditions can lead to key equipment failures like reactors, tanks, and heat exchangers. Cor corrosion-resistant steel alloys are often employed to mitigate rusting, and protective coatings are applied to vulnerable areas. Proper maintenance and regular cleaning are critical to extend the life of equipment and ensure safety.
c. Marine Engineering
Steel is exposed to constant saltwater in marine environments, accelerating the rusting process. Ships, offshore platforms, and underwater pipelines made from steel are especially susceptible to corrosion. Stainless steel, coated carbon steel, and even specialized alloys like duplex stainless steel are used to improve rust resistance in these settings. Furthermore, applying anti-corrosion coatings and conducting regular inspections for pitting and crevice corrosion are vital practices in marine engineering.
6. How to Prevent Steel from Rusting?
Preventing rust on steel requires a combination of proper material selection, protective coatings, and regular maintenance. Here are some key strategies for preventing rust:
a. Use Corrosion-Resistant Steels
The first line of defense is choosing the proper steel grade for the environment. For instance, stainless steel or alloy steel may be a better choice in corrosive environments than carbon steel. Ensure that the steel’s properties align with the industry’s demands.
b. Apply Protective Coatings
Coatings like paint, galvanization (zinc), or specialized corrosion-resistant coatings can protect steel from rusting. These coatings are particularly important for environments with high salt exposure, such as marine engineering or coastal oil rigs.
c. Regular Maintenance and Inspections
Routine cleaning and inspection are essential in preventing rust. This involves removing dirt, chemicals, and moisture from the surface and checking for early signs of corrosion. If rust is detected, immediate action should be taken, such as sanding, recoating, or replacing affected parts.
d. Control Environmental Factors
Where possible, reduce exposure to moisture, chemicals, and salts. In industries such as chemical processing, ensure that steel structures are protected from aggressive chemicals that can accelerate rusting. In marine settings, consider using sacrificial anodes or installing cathodic protection systems.
7. Conclusion
While steel is an incredibly durable material, it is not immune to rusting. Understanding the factors that cause steel to rust and selecting the appropriate material and maintenance practices is crucial for ensuring the longevity and safety of steel structures, especially in industries like oil and gas, chemical processing, and marine engineering.
By proactively managing rust risks through proper material selection, coatings, and regular inspections, companies can significantly extend the lifespan of their steel infrastructure and reduce the likelihood of costly repairs or dangerous failures. Always consult a materials expert to ensure the right solution for your specific application, and keep an eye on the environmental factors that could compromise steel’s performance over time. Rust doesn’t have to be inevitable if the proper precautions are taken.




