Microstructure Evolution of P92 Steel at Different Isothermal Temperatures
Microstructure Evolution of P92 Steel at Different Isothermal Temperatures
P92 steel is mainly used in ultra-supercritical boilers, ultra-high-pressure pipelines, and other high-temperature and high-pressure equipment.P92 steel is in the P91 steel chemical composition based on the addition of trace elements of W and B elements, reduces the content of Mo, through the grain boundaries of the strengthened and dispersion strengthened in a variety of ways, to improve the comprehensive performance of the P92 steel, P92 steel than the P91 steel has better resistance to oxidation performance and corrosion resistance. A hot working process is essential for producing the P92 steel pipe. Thermal processing technology can eliminate the internal defects generated in the production process and make the steel performance meet the needs of working conditions. The type and state of the organization in the hot working process are the key factors influencing the performance to meet the standard. Therefore, this paper analyzes the organization of P92 steel pipe at different isothermal temperatures to reveal the organization evolution of P92 steel pipe at various temperatures, which not only provides information support for the organization analysis and performance control of the actual hot working process but also lays the experimental basis for the development of the hot working process.
1. Test Materials and Methods
1.1 Test Material
The tested steel is a P92 steel pipe in use condition (1060 ℃ hardened + 760 ℃ tempered), and its chemical composition is shown in Table 1. A cylindrical specimen of ϕ4 mm × 10 mm was cut in the middle part of the finished pipe at a particular position along the length direction, and the quenching expansion meter was used to study the tissue transformation at different temperatures.
Table 1 Main Chemical Composition of P92 Steel by Mass Fraction (%)
| Element | C | Si | Mn | Cr | Ni | Mo | V | Al | B | Nb | W | Fe |
| % | 0.13 | 0.2 | 0.42 | 8.67 | 0.25 | 0.48 | 0.19 | 0.008 | 0.002 | 0.05 | 1.51 | Balance |
1.2 Test Process
Using L78 quenching thermal expansion meter, 0.05 ℃ / s warming up to 1050 ℃ insulation 15min, 200 ℃ / s cooling down to room temperature. Measure the critical point of phase change of the material Ac1 is 792.4℃, Ac3 is 879.8℃, Ms is 372.3℃. The specimens were heated up to 1050°C at a rate of 10°C/s and held for 15 min, and then cooled down to different temperatures (770, 740, 710, 680, 650, 620, 520, 430, 400, 370, 340, 310, 280, 250, 190, and 160°C) at a rate of 150°C/s and held for different periods of time (620°C and below for 1h, 620°C and above for 25h). 620 ℃ and above holding 25h), the isothermal end of the power is off so that the specimen is air-cooled to room temperature.1.3 Test methods
After grinding and polishing the surface of the specimens under different processes, the surface of the specimens was corroded using aqua regia. AXIOVERT 25 Zeiss microscope and QWANTA 450 environmental scanning electron microscope were used to observe and analyze the organization; using HVS-50 Vickers hardness tester (load weight of 1kg), hardness measurements were made at several locations on the surface of each specimen and the average value was taken as the hardness value of the specimen.
2. Test Results and Analysis
2.1 Organization and Analysis of Different Isothermal Temperature
Figure 1 shows the microstructure of P92 steel after complete austenitization at 1050°C for different times at different temperatures. Figure 1(a) shows the microstructure of P92 steel after isothermalization at 190℃ for 1h. From Fig. 1(a2), it can be seen that its room temperature organization is martensite (M). From Fig. 1(a3 ), it can be seen that the martensite shows lath-like characteristics. Since the Ms point of the steel is about 372°C, the martensite phase transformation occurs at isothermal temperatures below the Ms point, forming martensite, and the carbon content of the P92 steel belongs to the range of low carbon compositions; a lath-like morphology characterizes the martensite.
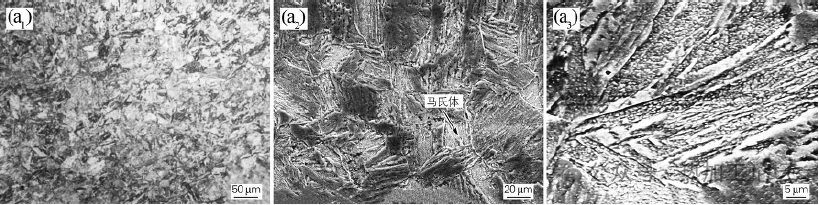
Figure 1(a) shows the microstructure of P92 steel after 1h isothermal at 190°C
Figure 1(b) for the microstructure of P92 steel at 430 ℃ isothermal 1h. As the isothermal temperature increases to 430°C, P92 steel reaches the bainite transformation zone. Since the steel contains Mo, B, and W elements, these elements have little effect on the bainite transformation while delaying the pearlitic transformation. Therefore, P92 steel at 430 ℃ insulation 1h, the organization of a certain amount of bainite. Then the remaining supercooled austenite is transformed into martensite when air-cooled.

Figure 1(b) for the microstructure of P92 steel at 430 ℃ isothermal 1h
Figure 1(c) shows the microstructure of P92 steel at 520 ℃ isothermal 1h. When the isothermal temperature of 520 ℃, the alloying elements Cr, Mo, Mn, etc., so that the pearlite transformation is inhibited, the start of the bainite transformation point (Bs point) is reduced, so in a specific range of temperatures will appear in the stabilization zone of the supercooled austenite. Figure 1(c) can be seen in 520 ℃ insulation 1h after supercooled austenite did not occur after the transformation, followed by air cooling to form martensite; the final room temperature organization is the martensite.

Figure 1(c) shows the microstructure of P92 steel at 520 ℃ isothermal 1h
Figure 1 (d) for the P92 steel at 650 ℃ isothermal 25h microstructure for martensite + pearlite. As shown in Figure 1(d3), pearlite shows discontinuous lamellar characteristics, and the carbide on the surface shows a short rod precipitation. This is due to the P92 steel alloying elements Cr, Mo, V, etc. to improve the stability of supercooled austenite at the same time so that the P92 steel pearlite morphology changes, that is, the carbide in the pearlitic body of the carbide for the short rod, this pearlitic body is known as the class pearlite. At the same time, many fine second-phase particles were found in the organization.
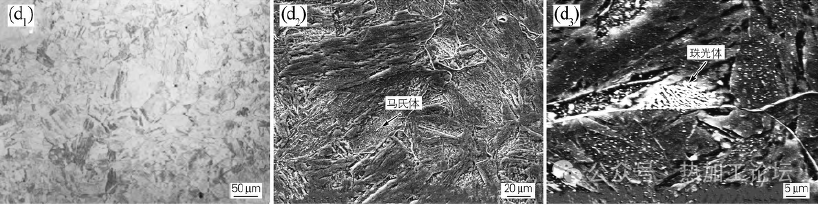
Figure 1 (d) for the P92 steel at 650 ℃ isothermal 25h microstructure for martensite + pearlite
Figure 1(e) shows the microstructure of P92 steel at 740 ℃ isothermal 25h. At 740°C isothermal, there will be first eutectic massive ferrite precipitation and then austenite eutectic decomposition, resulting in pearlite-like organization. Compared with the 650°C isothermal (see Fig. 1(d3)), the pearlitic organization becomes coarser as the isothermal temperature is increased, and the two-phase character of pearlite, i.e., ferrite and carburite in the form of a short bar, is clearly visible.
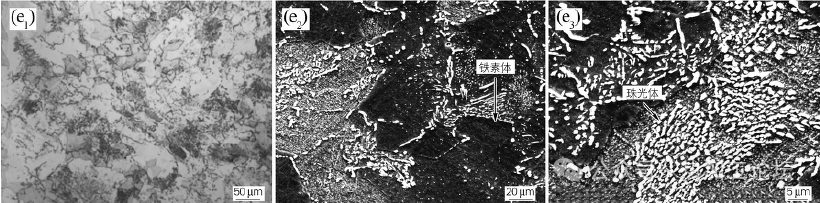
Figure 1(e) shows the microstructure of P92 steel at 740 ℃ isothermal 25h
Fig. 1(f) shows the microstructure of P92 steel at 770°C isothermal temperature for 25h. At 770°C isothermal, with the extension of the isothermal time, the precipitation of ferrite occurs first, and then the supercooled austenite undergoes eutectic decomposition to form a ferrite + pearlite organization. With the increase of isothermal temperature, the first eutectic ferrite content increases, and the pearlite content decreases. Because of the P92 steel alloying elements, alloying elements dissolved into the austenite to make the austenite hardenability increase, the difficulty of the eutectic decomposition becomes more extensive, so there must be a sufficiently long isothermal time to make its eutectic decomposition, the formation of the pearlitic organization.
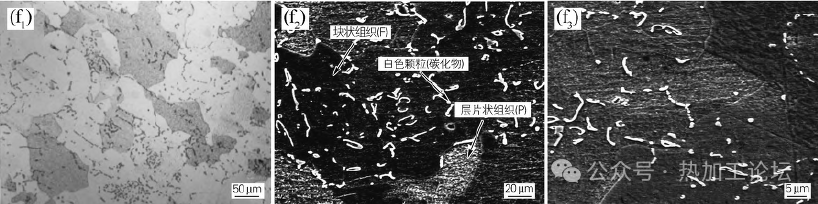
Fig. 1(f) shows the microstructure of P92 steel at 770°C isothermal temperature for 25h
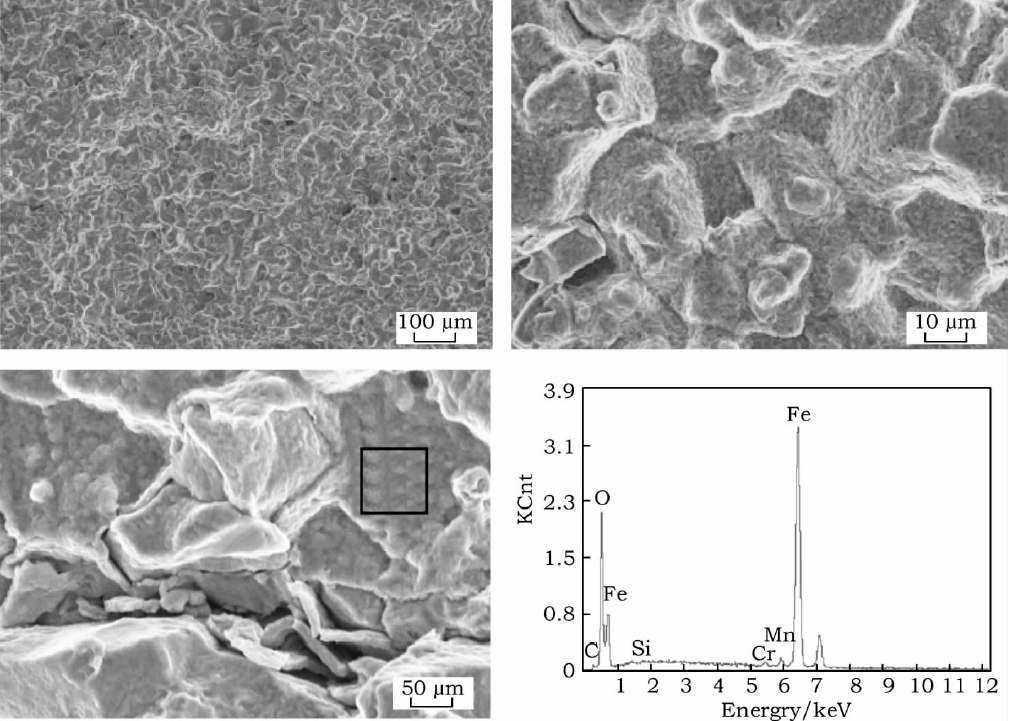
Energy spectrum analysis was performed on the tissues with different morphologies in Fig. 1(f2) to identify the tissue type further, as shown in Table 2. From Table 2, it can be seen that the carbon content of the white particles is higher than other organizations, and the alloying elements Cr, Mo, and V are more, analyzing this particle for the composite carbide particles precipitated during the cooling process; comparatively speaking, the carbon content in the discontinuous lamellar organization is second to the lowest, and the carbon content in the massive organization is the least. Because pearlite is a two-phase organization of carburize and ferrite, the average carbon content is higher than that of ferrite; combined with isothermal temperature and morphology analysis, it is further determined that the lamellar organization is pearlite-like, and the massive organization is first eutectic ferrite.
Spectrum Analysis Of The P92 Steel, Isothermally Treated At 770 °C For 25 Hours, Written In Table Format With Atom Fractions (%)
| Structure | C | Nb | Mo | Ti | V | Cr | Mn | Fe | W |
| White Granules | 11.07 | 0.04 | 0.94 | 0.02 | 2.16 | 8.36 | 2.64 | 54.77 | 2.84 |
| Block Structure | 9.31 | 0.04 | 0.95 | 0.2 | 0.32 | 8.42 | 0.74 | 85.51 | 10.21 |
| Layered Structure | 5.1 | 0 | 0.09 | 0.1 | 0.33 | 7.3 | 0.35 | 85.65 | 0.69 |
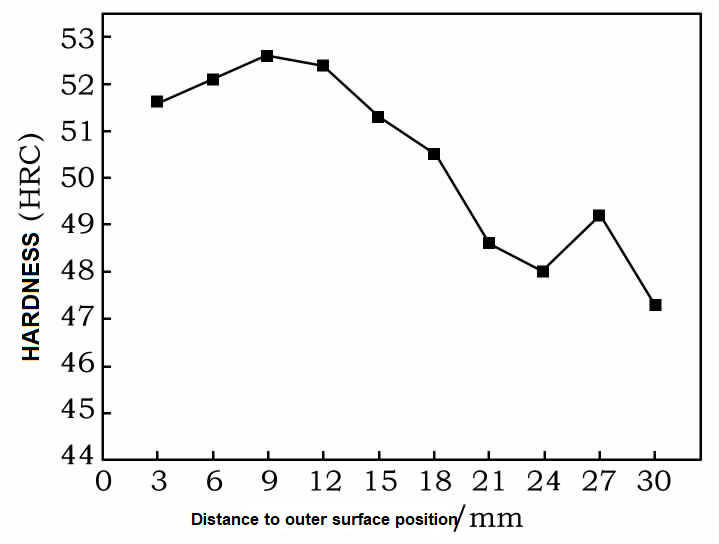
2.2 Microhardness and Analysis
Generally speaking, during the cooling process of alloy steels containing elements such as W and Mo, three kinds of organizational transformations occur in the supercooled austenite: martensitic transformation in the low-temperature zone, bainite transformation in the medium-temperature zone, and pearlite transformation in the high-temperature zone. The different organizational evolutions lead to different hardnesses. Figure 2 shows the variation of the hardness curve of P92 steel at different isothermal temperatures. From Fig. 2, it can be seen that with the increase of isothermal temperature, the hardness shows the trend of decreasing first, then increasing, and finally decreasing. When the isothermal temperature of 160 ~ 370 ℃, the occurrence of martensitic transformation, Vickers hardness from 516HV to 457HV. When the isothermal temperature is 400 ~ 620 ℃, a small amount of bainite transformation occurs, and the hardness of 478HV increases to 484HV; due to the small bainite transformation, the hardness does not change much. When the isothermal temperature is 650 ℃, a small amount of pearlite forms, with a hardness of 410HV. when the isothermal temperature of 680 ~ 770 ℃, the formation of ferrite + pearlite organization, hardness from 242HV to 163HV. due to the transformation of P92 steel at different temperatures in the organization of the transition is different, in the region of the low-temperature martensitic transformation, when the isothermal temperature is lower than the point of Ms, with the increase in temperature, martensite content decreases, hardness decreases; in the middle of the transformation of P92 steel in the different temperatures, when the isothermal temperature is lower than the Ms point, with the temperature increase, martensitic content decreases, the hardness decreases; in the medium-temperature bainite transformation region, because the amount of bainite transformation is small, the hardness does not change much; in the high-temperature pearlitic transformation region, with the rise of isothermal temperature, the first eutectic ferrite content increases so that the hardness continues to decline, so with the increase in isothermal temperature, the material hardness is generally a decreasing trend, and the trend of the change in hardness and the analysis of the organization is in line with the trend.
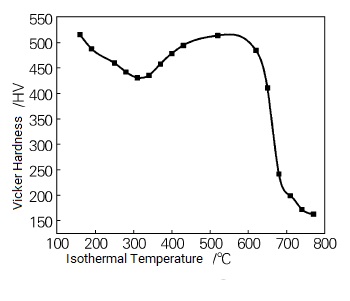
Variation Of Hardness Curves Of P92 Steel At Different Isothermal Temperatures
3. Conclusion
1) The critical point Ac1 of P92 steel is 792.4 ℃, Ac3 is 879.8 ℃, and Ms is 372.3 ℃.
2) P92 steel at different isothermal temperatures to obtain the room temperature organization is different; in the 160 ~ 370 ℃ isothermal 1h, the room temperature organization is martensite; in the 400 ~ 430 ℃ isothermal 1h, the organization of a small amount of bainite + martensite; in the 520 ~ 620 ℃ isothermal 1h, the organization is relatively stable, a short period of time (1 h) does not occur within the transformation, the room temperature organization is martensite; in the 650 ℃ isothermal 25h, the room temperature organization is pearlite. h, room temperature organization for pearlite + martensite; in 680 ~ 770 ℃ isothermal 25h, the organization transformed into pearlite + first eutectic ferrite.
3) P92 steel austenitization in Ac1 below isothermal, with the reduction of isothermal temperature, the hardness of the material as a whole tends to increase, isothermal at 770 ℃ after the occurrence of the first eutectic ferrite precipitation, pearlitic transformation, hardness is the lowest, about 163HV; isothermal at 160 ℃ after the occurrence of the martensitic transformation, hardness is the highest, about 516HV.